The pH level of water can affect fish in many different ways. Some fish are better off at a higher or lower pH, and some species will not survive if the pH is outside their normal range.
The most important thing you need to know is that altering the pH level of water too much causes stress on your fish’s bodies and could lead to death.
To ensure that your aquarium stays healthy for as long as possible, it’s very important to make sure the pH levels are within an acceptable range. You’ll also want to monitor ammonia, nitrite, nitrate, hardness & alkalinity levels because these conditions can cause major problems for your tank.
What pH level Will Kill Fish?
The ideal pH range for most freshwater fish is between 6.5 and 7.5, with a higher value being better for some species (such as goldfish). If you have an acidic tank that measures below 6.0, it may be time to do some water changes or add a buffer such as baking soda. On the other hand if you find yourself constantly battling high pH levels above 8, then it might be time to invest in a reverse osmosis filter or reduce carbonate hardness by adding calcium chloride tablets into your tank.
How do I Adjust the pH in my Freshwater Aquarium?
The best way to achieve a neutral or slightly acidic pH level (6-7) in a freshwater aquarium is by using aquarium buffer, which contains two types of chemicals: sodium bicarbonate and potassium bicarbonate. These chemicals are both buffering agents that help neutralize acids like carbon dioxide from being absorbed into the water column and creating drastic changes in your tank
How Does Low pH Affect Fish?
When the pH level drops below 7, the fish tank becomes more acidic. At this point, your fish could die or be seriously injured by environmental stress. This is why it’s important to monitor how much acidity there is in the water so that you can prevent any problems before they occur! The most common way for a change in ph levels to occur is through an overabundance of organic waste products from plants and algae.
Understanding the Ph Scale
The pH scale, which was introduced by S. P. Sørensen in 1909, runs form 1 to 14 and expresses how acid or alkaline a solution is. pH comes from the French pouvoir hydrogène, and means “hydrogen power”.
The numbers equate inversely to the concentrations of hydrogen (H+) and hydroxide (OH–) ions in a solution.
A pH of 1 means that 10-1, or one tenth of a gram, of hydrogen is dissolved in one litre of solution; a pH of 2 means that 10-2 or one hundredth of a gram, of hydrogen is dissolved in one litre of solution and so on. The more hydrogen ions, the more acidic the water and the lower the pH value.
A solution, which contains equal concentrations of hydrogen and hydroxide ions, such as pure water, is termed “neutral” and has a pH of 7.
A value of below 7 on the pH scale is termed “acid” and indicates a concentration of hydrogen ions. Conversely a concentration of hydroxide ions would return a value of above 7 and is termed “alkaline”.
The scale is logarithmic; that is each step up or down is 10 times that of the previous one. Two steps are 100 times change, three steps are 1000 times change and so on.
Graph
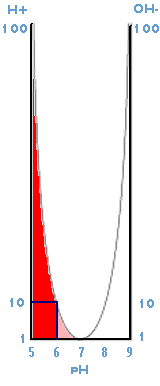
The graph illustrates the ratio of hydrogen to hydroxide ions between pH 5 and 9.
- At pH 7 the ratio is 1:1 and therefore neutral.
- At pH 6 the ratio is 10:1 Ten times more acidic than pH 7.
- At pH 5 the ratio is 100:1 One hundred times more acidic than pH 7
- Past pH 7, the ratio favours hydroxide ions, thus; at pH 8 the ratio is 1:10 and at pH 9 the ratio is 1:100
In all, the graph indicates the ten thousand times difference between pH 5 and pH 9.
So how does aquarium water pH affect the fish keeper?
90% of all fresh water fish exist in water with a pH of 5.5 – 7.5 and African Lake cichlids extend this range to around pH 8.5.
As explained above, this range represents a variation of over 1000 times the concentration of hydrogen ions so even an apparently small change in pH can have a dramatic effect on the fish causing stress or even death.
Some fish can tolerate a range of pH values if acclimatised slowly but always research the fish that you intend to keep as some fish are more susceptible to a change in pH than others.
Measuring pH can be a good way of monitoring when water changes are required. Acid conditions can often result from an excess of waste products (producing carbonic acid) in the aquarium and a surplus of wastes can weaken fish eventually causing disease and death.
In a well established tank it is possible this can create a natural acid buffer which will resist changes to a more acceptable pH. If this begins to occur, check and see if the substrate is abnormally dirty or if using an undergravel filter, check it is not clogged.
The pH of water is also closely associated with hardness. The calcium salts which harden water, such as those contained in limestone, also render it alkaline; by contrast soft water is often acidic. This is worth remembering if trying to modify the pH.
There is little point in trying to acidify water which is constantly being buffered back to neutral or alkaline by calciferous decor. First one must minimise the hardness.
Lowering pH in an aquarium – making water more acidic:
- Filter the water over peat.
- Make a partial water change using softer water. You can use rain water or water prepared by boiling.
- Add bogwood to the tank.
- Use a commercial pH minus (acid) buffer.
Raising pH – making water more alkaline:
- Aerate vigorously to expel carbon dioxide (CO2).
- Filter over coral, crushed shells or limestone chips. Add calciferous decor to the aquarium such as tufa rock or a coral sand substrate.
- Make a partial water change.
- Use a commercial pH plus (alkaline) buffer.
Any change to the pH should be undertaken gradually and particular care should be taken when using a commercial pH buffer. Often they create a rapid pH change and cause the fish to suffer pH shock. I recommend preparing water in advance and adding it slowly, perhaps during a water change.

